
Главная
страница
Сведения об
авторах
Study on Nitrogen Conversion into Oxides During Biomass Combustion
Plečkaitienė R., Buinevičius K.,
Kaunas University of Technology, Kaunas, LithuaniaAbstract
The European Union tightens norms referred to permissible pollution, so the objective of the work is to investigate the nitrogen oxides content in products of biomass and waste combustion as well as to calculate conversion of nitrogen contained in combustible material type into nitrogen oxides. The following fuel types referred to biomass and waste were investigated: wood granules, buckwheat hull, corn stalk granules, shredded packs, etc. The article contains combustion method description, experimental investigation results, comparison with permissible concentration norms and obtained ratios of nitrogen conversion into nitrogen oxides. During the investigation dependences of NOX concentration and nitrogen conversion coefficient on nitrogen content in combustible material were determined.
Introduction
Like other world countries, the European Union for some time past pays more attention to use of renewable energy sources, whereas fossil fuel sources have been depleted although energy-efficient means have been applied, high energy-saving technologies have been developed.
Whereas the European Union restricts permissible pollution norms (Project of Directive 2007), the objective of the work is to investigate the content of nitrogen oxides in biomass and waste combustion products as well as to calculate conversion of nitrogen contained in fuel type into nitrogen oxides.
Technique
The investigations were performed using a biomass combustion stand (Fig. 1) from Kaunas University of Technology (KUT) Heat and Nuclear Power Engineering Department.
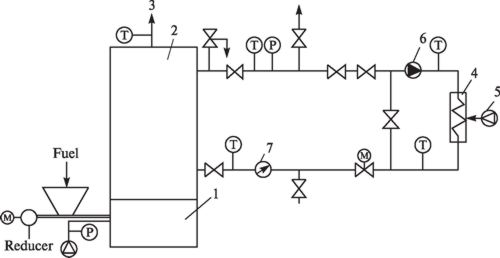
Fig. 1. Basic diagram of biomass
combustion stand:
1 — combustor; 2 — water boiler; 3 — chimney; 4 — heating unit; 5 — cooling
fan; 6 — circulation pump; 7 — heat counter
The biomass combustion stand includes the combustor 1 with the fuel and air feeders. The boiler 2 is installed above the combustor, combustion products are removed to the chimney 3. An outlet for smoke analysis and temperature measurements is installed in the chimney. Measurements are performed via ECOLINE 6000 gas analyzer. Heated water from the boiler is supplied via the pump 6 to the cooling system which consists of the heating unit 4 with the cooling fan 5. Air heated in the heating unit is removed outside, and cooled water from the circulation circuit is returned to the boiler.
The analysis of nitrogen contained in investigated materials was performed in LAI Agrochemical Research Centre; heat generation and ash content were investigated in the above-mentioned KUT Department (Table).
Characteristics of investigated fuel types
|
Fuel type |
N content, % |
Heat generation, kJ/kg |
Ash content, % |
|
Wood pellets |
0.067 |
17 025 |
0.39 |
|
Corn stalk pellets |
0.590 |
15 617 |
2.70 |
|
Buckwheat hull |
0.574 |
15 912 |
1.45 |
|
Cornstarch granules |
1.235 |
14 934 |
0.95 |
|
Shredded packs (tetrapacks) |
0.021 |
19 451 |
13.13 |
|
Scrap wood and wood pellets |
0.217 |
16 333 |
1.36 |
|
Straw (80 %) and fuel oil (20 %) pellets |
0.440 |
15 743 |
8.47 |
|
Lignin (80 %) and fuel oil (20 %) pellets |
0.870 |
18 182 |
15.08 |
|
Mixture of all fuel types (fuel oil, straw, diesel fuel, etc.) |
0.370 |
17 024 |
10.22 |
Nitrogen conversion into nitrogen oxides is calculated as a part of fuel nitrogen converted into nitrogen monoxide. General equations are used for calculation of conversion ratio (Buinevičius, Kazilevičius, 2004):
V0 = 0.00027·Qh + 0.0234, (1)
Vd = 0.00025·Qh + 0.9756, (2)
here: V0 — theoretical air volume, m3/kg; Vd — theoretical combustion product volume, m3/kg; Qh — the lowest value of heat generated by fuel, kJ/kg (this is considered the most important wood fuel energy parameter).
Ratio of nitrogen conversion into nitrogen oxides is calculated as follows (Buinevičius, 2009):
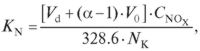
here: KN — fuel nitrogen conversion ratio, percentage; α — coefficient of excess air contained in combustion products (it depends on fuel type, combustion method, combustion facility structure and other factors); CNOx — concentration of nitrogen oxides in combustion products, mg/m3; NK — nitrogen content in fuel, percentage; 328.6 — conversion factor.
Results
There were several investigation objectives: determination of dependences of NOX concentration and nitrogen conversion coefficient on nitrogen content and calculation of the value of fuel nitrogen conversion into NOX, so that these results would be applicable for calculation of NOX emissions for other biomass types.
Values of NOX and CO measured during experiments did not exceed the limit of biomass fuel combustion product release permissible in Lithuania. Minimum CO concentrations were measured during combustion of scrap wood and wood pellets.
Concentration of nitrogen oxides escaping during waste combustion did not exceed specified permissible norms for waste in Lithuania, but in this case CO concentration limits were significantly exceeded. Maximum measured CO value reached 4 397 mg/m3. However, CO concentrations are technological parameters, and after changing of combustor, size of pellets or granules or air content permissible CO values can be obtained. During combustion of selected fuel types (Table) sulfur dioxide did not escape; an insignificant sulfur dioxide amount was observed in cases when material contained fuel oil additives.
After the analysis of experiments (Figs. 2 and 3) it was determined that NOX concentration depends on N content in combustible material: the more is nitrogen content in the fuel, the more combustion products release nitrogen oxides.
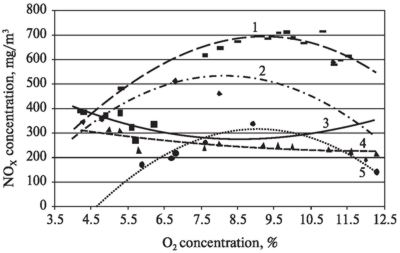
Fig. 2. NOX
concentration
dependence on oxygen concentration during biomass fuel combustion:
1 — cornstarch granules; 2 — corn
stalk pellets; 3 — buckwheat hull; 4 — scrap wood and wood pellets; 5 — wood
pellets
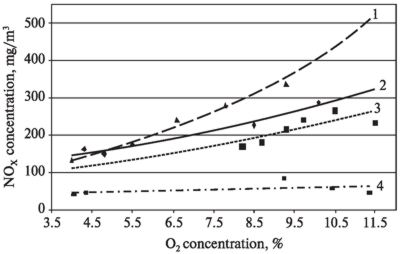
Fig. 3. NOX concentration
dependence on oxygen concentration during waste combustion:
1 — mixture of all fuel types; 2 —
lignin and fuel oil pellets; 3 — straw and fuel oil pellets; 4 — shredded packs
(tetrapacks)
Figure 4 provides nitrogen conversion dependence on nitrogen content in fuel. Calculations are performed applying formula (3). For the purpose of comparison the diagram includes KN values obtained during other investigations.
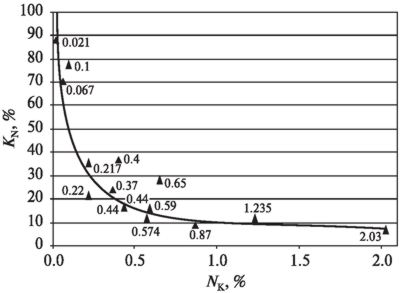
Fig. 4. Nitrogen part contained in the
fuel which is converted into nitrogen oxides:
point 0.021 obtained
during combustion of shredded packs; point 0.067 — during combustion of wood
pellets; point 0.217 — during combustion of scrap wood and wood pellets; point
0.37 — during combustion of mixture of all fuel types; point 0.44 — during
combustion of straw and fuel oil pellets; point 0.574 — during combustion of
buckwheat hull; point 0.59 — during combustion of corn stalk pellets; points
0.1; 0.4; 0.65 — during combustion of kerosene with pyridine (C5H5N)
additives (Buinevičius, Cirulnikov, 1990); points 0.22; 0.44 — during combustion
of natural gas with carbamide additives (Buinevičius, 2009); point 0.87 — during
combustion of lignin and fuel oil pellets; point 1.235 — during combustion of
cornstarch granules; point 2.03 — during combustion of dry pig manure (Čepanko,
Buinevičius, Pszczola, 2008)
The diagram shows that the more is nitrogen content in the fuel, the less part converts into nitrogen oxides.
The analysis of investigation results has showed that if there are totally different nitrogen sources and different types of fuel, contained nitrogen conversion into NOX can be generalized by the following equation:
KN
= 11.765·NK−0.6161, (4)here 11.765 - conversion factor.
Summary
1. Depending on oxygen NOX and CO have opposite change tendency. If CO increases, NOX concentration decreases.
2. Experiments have demonstrated that the more is nitrogen content in the fuel, the less part converts into nitrogen oxides. For example: if nitrogen content in fuel is about 2 %, about 8 % of nitrogen is converted into NOX. However, if nitrogen content in combustible is 0.2 %, about
З0 % of nitrogen is converted into NOX. When nitrogen content decreases, i.e. < 0.1 %, conversion degree reaches 90 %.3.
The equation has been derived to calculate nitrogen conversion into NOX.Исследование конверсии азота в оксиды при горении биомассы
Плечкайтене Р., Буйнявичюс К.,
Каунасский политехнический институт, Каунас, ЛитваЕвропейский союз постоянно ужесточает экологические нормы. Цель данной работы
— определение концентрации оксидов азота в продуктах горения, а также степени конверсии азота топлива. Исследовали следующие материалы: древесные гранулы; гречневую шелуху; пеллеты из стебля кукурузы; дробленые пакеты ТетраПак; пеллеты из смеси соломы и мазута и т. д. Описана методика сжигания топлива, приведены результаты экспериментов и сравнение с установленными нормами выбросов. Определена зависимость степени конверсии азота в оксиды от его содержания в сжигаемом материале.Plečkaitienė
Raminta, Lecturer, Kaunas University of Technology, K. Donelaičio St. 73, Kaunas,
LT-44029, Lithuania. Tel. (606) 5-49-98. E-mail
Буйнявичюс
Kястутис, д-р техн. наук, доц., Каунасский технологический университет, ул. K.
Донелайчио, 20, Каунас, 44239, Литва. Тел./факс (37) 30-04-41.
E-mail
© Независимое
агентство экологической
информации
Последние изменения внесены
06.04.11