Ãëàâíàÿ
ñòðàíèöà
Äîêëàäû
Ñâåäåíèÿ îá
àâòîðàõ
Biological Treatment of Wastewater by Algae: A Case Study of Copper Biosorption by Sargassum angustifolium
Naser Jafari, University of Mazandaran, Babolsar, Iran
Salman Ahmady-Asbchin, University of Ilam, Ilam, Iran
Abstract
In this study, the batch removal of Cu2+ ions from an aqueous solution and wastewater using marine brown alga Sargassum angustifolium (SA) was investigated. Desorption experiments indicated that CH3COOH and EDTA were efficient desorbents for recovery from Cu2+. With high capacities of metal biosorption and desorption, the biomass of SA is promising as a cost-effective biosorbent for the removal of Cu2+ from wastewater. According to X-ray photoelectron spectroscopic and Fourier transform infrared spectroscopic analysis, the possible organic functional groups in the metal binding include carboxyl, amino, sulfhydryl and sulfonate groups. The point of zero net proton charge (PZNPC) was found close to pH 6.1. The biosorption of copper ions and effect of pH was studied. All these observations indicate that the copper biosorption on SA is mainly based on ion exchange mechanism. During the ion exchange process, calcium, magnesium, hydrogen cations, and probably other cations (sodium and potassium) in the algal cell wall matrix were replaced by the tested copper ions. Langmuir, isotherm model were applied to describe the biosorption of the Cu (II) ions onto Sargassum biomass. The maximum adsorption capacity has been extrapolated to 0.94 mmol/g. This study demonstrated that the biomass of SA could be used as an effective biosorbent for the treatment of copper (II) containing wastewater streams.
Keywords: Biological treatment, alga, heavy metal, biosorbent.
1. Introduction
Heavy metals are major pollutants to the environment because of their toxicity and threat to plants, animals and human beings. Heavy metal are used in operations various industries such as the semiconductor industry. As a result, waste streams from the industries contain metal ions. Many studies have shown that they are highly toxic and can seriously damage our aqueous environment. Copper is recognized to be one of the most widespread heavy metal contaminants in the environment (Ho et McKay, 2003). The effects of heavy metals on functions of ecosystems vary considerably and are of concern to the economy and public health. In cases, governments may consider it necessary to remove some pollutants from the ecosystem; however, the economic cost of treatment may be very high, based on volume, concentration of metals, and salinity of wastewaters, making it impossible to use conventional technologies, such as precipitation and sludge separation, chemical oxidation or reduction, ion exchange, reverse osmosis, electrochemical treatment, and evaporation. In the last two decades, biosorption has drawn attention because of its low cost, sometimes high efficiency, and minimization of chemical or biological sludge. According to Gadd (2008), biosorption may be simply defined as the removal of substances, including heavy metal, from solution by biological material (biosorbent): It is a property of both living and dead organisms and their components. Biosorbents exhibit this property, acting just as a chemical substance, as ion exchanger of biological origin. Besides, biosorbents may be reused and the metals may be recovered (Kratochvil and Volesky 1997). Marine algal biomass is a good biosorbent due to its high uptake capacity and the ready abundance of biomass in many parts of the words. The biosorption capability of algae is attributed mainly to the cell wall, which contains various polysaccharides and other highly complex organic compounds. Metal sorption performance depends greatly on the chemistry of solutions. Solution pH plays an important role. It can change the nature of metal speciation and thus affect the sorption. With a higher pH, cationic metal ion uptake is generally enhanced. Among the biosorbents are some species of the brown alga Sargassum that have proved to be a highly effective biosorbent because of their capacity to remove high metals at a relatively rapid rate (Davis et al. 2000). In the Gulf of Persian of Iran, the genus Sargassum is an untapped resource that could be used for treating effluents from mining activity. This brown macroalgae showed a tendency to incorporate higher concentrations of elements from marine environments than red and green algae present in the region Bushehr near of Persian Gulf. To develop a biosorption process by using the SA for industrial applications, it is desirable to better understand the metal uptake process (Taboada-Serrano et al., 2005, Dambies et al., 2001)). Advanced instruments such as X-ray, photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FT-IR) can provide good tools to achieve the goal because they can provide insight into environmental process at the molecular level. XPS and FT-IR have been adopted to identify the major functional groups in a biopolymer and coordination types between the metal ions and the surface ligands (Figueria et al., 1999, Buschman and Sigg, 2004, Dambies et al., 2001). For example, the involvement of ion exchange, sorption and reduction was determined in the biosorption of Cu (II) on chitosan according to XPS analysis. An ion exchange model was developed and used to illustrate the metal biosorption onto the protonated Sargassum (Schiewer and Volesky 1996). In present work the SA was used to remove Cu (II) from aqueous solution. A series of experiments was first conducted to obtain the biosorption properties of biosorbent. Instrument analysis by FT-IR and XPS was performed to obtain the chemistry in the metal ions and functional groups of biosorbent were involved. The changes in the sorption chemistry were then compared. An equilibrium model capable of identifying the important sorption chemistry was developed, which was subsequently used to predict the biosorption process as a function operation parameters.
The aim of the present study was to characterize the nature and binding mechanism of chemical groups occurring in the brown alga SA that were responsible for copper biosorption. In addition the effects of initial metal ion concentration, contact time, concentration of algal biomass and pH.
2. Materials and methods
2.1. Seaweed
The raw biomass of SA was harvested from the Persian Gulf on Bushehr city. The biomass was first washed several times with deionized water to remove impurities, and then dried overnight in an oven with the temperature controlled at 60°C. The dry biomass was milled and an average of 0.3 mm size particles was used for biosorption experiments. Copper solutions of different concentrations (0.01 to 0.44 mmol/L) were prepared by adequate dilution of the stock solution with deionised water. All the adsorption experiments were carried out at room temperature (25 ± 1)°C. The initial pH was adjusted with 1 M HCl or 1 M NaOH. Single-metal concentrations in the relevant samples were determined by an atomic absorption spectrophotometer (Chem., Tech, Analytical CTA 2000). The liquid phase was separated from the adsorbent by a filtration system using 0.45 μm membranes.
2.2. Potentiometric titration
The potentiometric measurements were carried out at (25 ± 1)°C, using a glass electrode and under nitrogen stream to avoid dissolution of carbon dioxide in the solution. The equipment used consisted in a tetrameter with an automatic burette. A 0.1 g sample of protonated biosorbent Sargassum was first stirred in 100 ml of 0.001, 0.01 and 0.1 M NaCl solutions until the pH remained constant (2 h). From this curve, the point of zero net proton charge (PZNPC) was determined.
2.3. Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy was used to determine the changes in vibration frequency in the functional groups in fresh, protonated, and metal-loaded biosorbents. The spectra were collected by an FT-IR spectrometer (Hartman – Bomen) within the wavenember range of 400–4 000 cm−1. Specimens of various biosorbents (dry SA) were first mixed with KBr and then ground in an agate mortar (Merck, for spectroscopy) at an approximate ratio of 1/100 for the preparation of pellets (weight of 100 mg) the background obtained from the scan of pure KBr was automatically subtracted from the sample spectra. All spectra were plotted using the same scale on the absorbance axis.
2.4. Batch biosorption experiments
A series of copper biosorption experiments was conducted; the factors in the investigation included pH, temperature and adsorption capacity. The data were subsequently used for the model development as well as its validation. In the pH effect experiment, the desired solution pH was first adjusted by HNO3 or NaOH. The Sargassum was added to the solutions while being shaken at 1500 rpm in the orbital shaker. The experiment was performed at room temperature of (25 ± 1)°C. The pH was frequently measured and adjusted accordingly by HNO3 or NaOH. After the experiments, the supernatants were taken from each flask, acidified and filtered.
In the isotherm experiments were carried out in bottle flasks filled with 1000 ml of water thoroughly mixed with 0.1 g of Sargassum at (25 ± 1)°C. The initial concentrations of metal ions were ranged from 0.01 to 0.44 mmol/L. The initial pH was measured and if necessary, NaOH or HCl solution was added to reach an initial pH close to 6. The metal uptake at equilibrium was calculated by the following equation:
 (Eq. 1)
(Eq. 1)
where qe is the metal uptake (mg copper adsorbed per g adsorbent ),V is the solution volume, W is the amount of sorbent, Ci and Ce are the initial and equilibrium metal concentration in solution, respectively.
2.5 Desorption experiment
For the desorption study, contact made between 0.1 g dried biomass and a 100 ml copper solution (3 mmol/L). After copper ion sorption, the biomass was filtered, washed three times with distilled water to remove residual Cu(II) on the surface, and kept in contact with the 100 ml desorbent solution: HNO3, Ca(NO3)2, EDTA, CH3COOH and distilled water. The mixtures were shaken in a rotary shaker for 18 h. The filtrates were analyzed to determine the concentration of Cu (II) after desorption.
3. Results and Discussion
3.1. Potentiometric titration
As shown in Fig. 1, protonated Sargassum has a weak acid property. Some hydrogen ions are released from the biosorbent to neutralize the hydroxide when the NaOH is added. Before the sodium hydroxide was added, the pH values were 3.5 for the solution containing the Sargassum. It can be found that the biosorbent feature several pH values.

Fig. 1. Potentiometric titration of raw SA
3.2. Biosorption isotherms
Figure 2 shows the copper uptake isotherms at pH 5.0. The experimental results were corrected with the Langmuir isotherm model. The Langmuir adsorption isotherm is probably the most widely applied adsorption isotherm. This model which is valid for monolayer sorption onto a surface with a finite number of identical sites which are homogeneously distributed over the sorbent (Xiangliang et al. 2005)
 (Eq. 2)
(Eq. 2)
For determining the equilibrium parameters, Eq. (2) can be altered into a linear from as follows:
 (Eq. 3).
(Eq. 3).
The values of qm and b were obtained from Eq. 3. The linear correlation coefficient was 0.993 and qm was 0.94 mmol/g from calculation using the Langmuir equation.

Fig. 2. Sorption isotherm of a Copper in demonized water (●) and in tap water (▲)
Experimental fixation capacities obtained in tap water are much less described by the tested models and show a decrease in comparison with deionised water. The difference could be attributed to a competition mechanism between ions naturally present in tap water and copper, for the binding moieties present onto Sargassum surface. This indicates that, in addition to the ion exchange mechanism, there are other reactions contributing to the heavy metal binding. The adsorption study has highlighted an ion exchange mechanism responsible for metal uptake. The release of calcium, initially fixed onto the Sargassum, has been followed in the same time of copper adsorption. This release depends on the initial copper concentration of the solution, which could lead to a fixation mechanism by ion exchange. Because the isotherms of Cu2+ adsorption and Ca2+ desorption were practically similar, Cu2+ ions seemed to be exclusively adsorbed by an ion exchange mechanism. Ion-exchange is an important in biosorption, because it explains many of the observations made during heavy metal uptake experiments. Under certain conditions, the ions attracted to a solid surface may be exchanged with other ions in an aqueous solution. Both cations and anions exchange can occur, but in some natural material, cations exchange is the dominant process.
3.3. Desorption experiment
Figure 3 shows the percentage of Cu (II) released by SA pieces after treatment with different desorbents. It was observed that the percentage of desorption using distilled water was almost negligible.

Fig. 3. Copper ions recovered by different desorbents
The desorption percentage decreased with a decreasing concentration of HNO3 (0.1 mol/L HNO3 : 56%; 0.01 mol/L HNO3 : 42%). Chelating agent EDTA was more efficient than HNO3 and Ca(NO3)2, and 0.01 mol/L EDTA can remove 79% of Cu (II) bound to biomass. CH3COOH was more efficient than other desorbents, and 0.1 mol/L CH3COOH can remove 89% of copper bound to biomass. This is attributed to the high value of the conditional formation constant of the complex Cu–CH3COOH (kf = 1.14·1010, pH = 5.0), which favors the desorption of Cu from the biomass. The high recovery percentage of Cu (II) by CH3COOH and EDTA allows the recycling of Cu from the biomass in industry.
3.4. Effect of pH on biosorption
The effect of pH on copper biosorption on Sargassum biomass is studied at room temperature by varying the pH of copper solution. The present results indicate that in the absence of the biomass a chemical precipitation occurred since the copper began to precipitate after pH 5.5 (Fig 4.).

Fig. 4. Effect of pH on the copper biosorption by SA
The pH is one of the most important parameters of biosorption (Kapoor et al. 1999; Aksu, 2001) and, regarding Sargassum sp., its high content of ionizable groups (carboxyl groups), makes it very liable to be influenced by the pH of the medium. For Sargassum sp., carboxyl groups are chiefly responsible for binding metallic ions. However other functional groups may contribute to the process, such as the sulfonate and amine groups. The higher the pH value, the higher the dissociation since free sites for the binding of copper can be produced; however, the majority of heavy metals precipitate at pH values over 5.5.
3.5. Fourier transform infrared spectroscopy
The FT-IR analysis was used for detecting vibration frequency changes of native moieties in the algal sorbent. Infrared spectra of Sargassum biomass samples before and after copper binding are shown in Fig. 5.
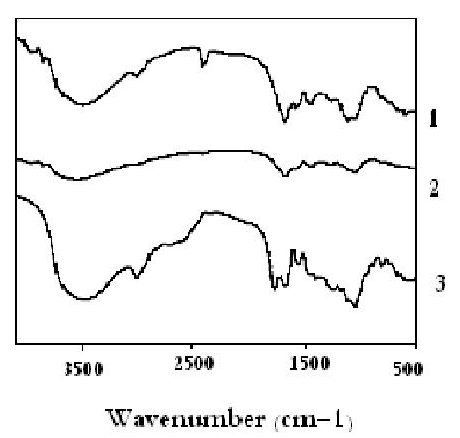
Fig. 5. FT-IR spectra of raw Sargassum (1), Sargassum-Cu (2), Sargassum protonated (3)
The copper Sargassum displayed new absorbance bands at 876, 779, 693, and 466 cm−1. The spectra of both samples displayed absorbance bands at approximately 1 630, 1 420, and 1 085 cm−1, but the band intensities of the nickel-loaded Sargassum were significantly higher. These bands indicated stretching of the carbon–oxygen bonds in the carboxyl groups and the nitrogen – hydrogen bond in amino groups, respectively (Fourest and Volesky, 1996; Ashkenazy et al., 1997; Kokinos et al., 1998).
3.6. Determination of the point of zero net proton charge
The titrations of the Sargassum previously placed in different ionic strengths solutions and conducted with HCl and NaOH 0.1 M are shown in Fig. 6. The pH of the solution affects the surface charge of the adsorbent, the degree of ionization, and the speciation of the surface functional groups. From the curves, the ph value for the zero net surface charge can be deduced close to 6.1 ± 0.2.
As the pH increase, a potential enhancement in metal removal can be explained by the decrease in competition between protons and metal cations for the same functional groups and the decrease in positive surface charge, which results in a lower electrostatic repulsion between the surface and the metal ions.
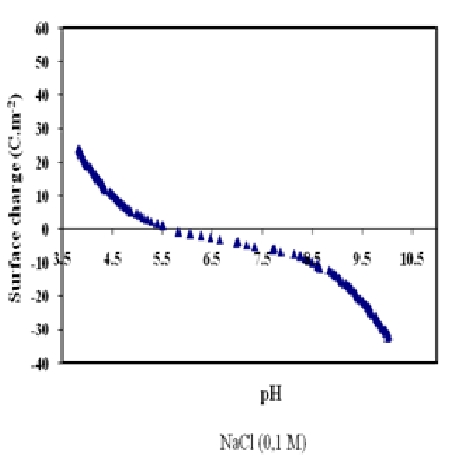
Fig. 6. Graphic determination of the PZNPC
3.7. X-ray Photoelectron Spectroscopy Analysis (XPS)
Table I shows the atomic concentrations of Cu2+ in the metal-laden Sargassum samples, according to the XPS spectra analysis. It's also shows changes in the atomic concentration in the functional groups after the metal binding process (values in brackets). The changes in algal biomass observed after metal uptake included increases in carbon and sulfur atomic concentrations and decreases in nitrogen, oxygen, and calcium and magnesium atomic concentrations. After Cu2+ uptake, there were significant changes in the atomic concentration of oxygen, carbon and nitrogen and almost no change in sulfur atomic concentrations.
Table 1
XPS atomic concentration (in percentage) of relevant chemical elements in SA sample before and after heavy metal uptake
|
Atom |
No metal uptake* |
Cu** |
|
C |
47.44 |
59.24 (+24.89) |
|
O |
38.4 |
24.18 (−37 ) |
|
N |
3.52 |
1.2 (−65.9) |
|
Ca |
4.99 |
– (−100) |
|
Mg |
4.53 |
1.4 (−69.1) |
|
S |
1.12 |
3.98 (+249) |
|
Cu |
– |
10 |
*Raw Sargassum sample.
**Values in brackets represent changes in XPS atomic concentrations after metal uptake by SA biomass.
These findings indicate that heavy metal uptake is accompanied by changes in sulfur, nitrogen, oxygen and carbon binding.
4. Discussion
This study indicates that brown marine algae SA, which is widely available at a low cost, can be used as an efficient biosorbent material for the treatment of Cu2+ in wastewater. The adsorption isotherm of Cu2+ by dried SA pieces could be adequately described by the Langmuir isotherm model. The maximum adsorption capacity was 0.94 mmol/g. Desorption experiments proved that CH3COOH and EDTA were an efficient and practical desorbents for the recovery of Cu2+ from the biomass. The pH value that was selected for the experiments on the biosorption of copper by SA was pH 5 since it combined the best characteristics for the lowest chemical precipitation and the highest biosorption. A large number of acidic functional groups at the surface leads to an high value of total proton exchange capacity and a copper uptake by cationic exchange mechanism. With advantages of high metal biosorption and desorption capacities, the biomass of Sargassum is a promising application as a cost-effective biosorbent material for the removal of copper from wastewater. The comparison of various literature studies of copper removal by biosorption showed in table 2.
Table 2
Comparison of various literature studies of copper removal by biosorption
|
Species |
pH |
Ion |
Capacities (mmol/g) |
References |
| Fucus serratus | 5.5 |
Cu2+ |
1.73 |
Ahmady-Asbchin et al, 2008 |
| Sargassum vulgare | 4.5 | Cu2+ | 0.93 |
Davis et al. 2003 |
| Sargassum fluitans | 4.5 | Cu2+ | 0.80 | Davis et al. 2000 |
|
Codium vermilara |
6.0 | Cu2+ | 0.27 | Romera et al.2007 |
|
Spirogyra insignis |
6.0 | Cu2+ | 0.30 |
Romera et al.2007 |
|
Chondrus crispus |
6.0 | Cu2+ | 0.64 |
Romera et al.2007 |
| Palmaria palmata |
6.5–7.0 |
Cu2+ | 0.10 | Pracher et al. 2004 |
|
Aspergillus armata |
6.0 | Cu2+ | 0.33 |
Romera et al.2007 |
|
Aspergillus niger |
5.0 | Cu2+ | 0.24 | Dursun et al., 2003 |
Acknowledgments
This research was supported by the Ilam University of Iran.
Naser Jafari, PhD in Environmental Ecology, Assistant Professor, Department of Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran. Tel./fax +98 (112) 525-26-81. E-mail
